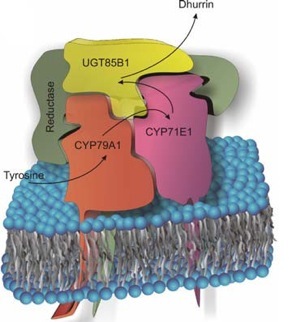
Figure 4A from the article: "Cyanogenic glycosides: a case study for evolution and application of cytochromes P450", Bak et al., 2006, Phytochem Reviews. The full publication is available here: link.springer.com.
Many bioactive natural products seem to be formed by enzymes that transiently form supramolecular complexes, whose structures allow for rapid synthesis of the final products. This subproject is devoted to investigating the formation and roles of these complexes.
In spite of the large number of different enzymes produced in a plant, their number fail to explain the huge chemical diversity observed in the plant kingdom. Some enzymes are therefore involved in more than a single pathway and in formation of more than a single product. This also applies for bio-active natural products where e.g the number of different glycosides found in a plant clearly exceeds the number of UDP-glycosyl transferases. Accumulating evidence suggests that many cellular reactions within metabolic pathways are catalyzed not by free-floating soluble enzymes, but via membrane-associated multienzyme complexes (metabolons). The formation of metabolons implies physical interaction between the protein constituents and requires that the components have an identical subcellular localization. Understanding how protein interactions and metabolon formation are mediated is an essential prerequisite to control the formation of the metabolons. This is a critical issue to understand and a prerequisite to optimize success in metabolic engineering approaches such as engineering plants to produce structurally complex pharmaceutical molecules in high concentrations.
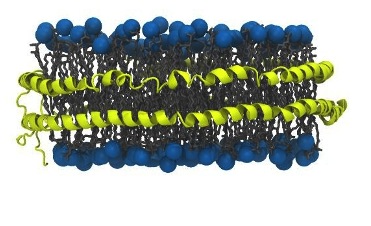
Illustration of a nanodisc comprised of a lipid bilayer disc encircled by two amphipathic membrane scaffold proteins (MSP) oriented in a double belt structure. Amphipathic molecules such as these can be used to handle functional membrane protein complexes in solution.
We hypothesize that metabolons are important to coordinate the biosynthesis of bio-active natural products in plants and that dynamic metabolons offer a mechanism for rapid reorganization of metabolite fluxes to optimize the physiological performance of the plant. We are therefore investigating innovative routes to isolate the putative dhurrin forming metabolon. We also study the role of specific types of phospholipids and of ER-resident proteins in forming or stabilizing the metabolon. The organization of the enzymes in the dhurrin biosynthesis pathway in sorghum is being used as an experimental model, and we are attempting to reconstitute the pathway in vitro using liposomes and purified recombinant sorghum proteins.
Principal Investigator
Tomas Laursen
tola@plen.ku.dk
+45 35 33 33 29
Grant Holder
 Birger Lindberg Møller
Birger Lindberg Møller
blm@plen.ku.dk
+45 3533 3352
